Abstract
Introduction Patients’ preferences are crucial to formulate a personalized treatment plan for cancer. We conducted a prospective, observational study with a novel, self-reported questionnaire, Therapy Preference Scale (TPS), to examine the effects of efficacy and safety outcomes, therapy burden and toxicities, and overall goals of care on patients’ preferences for treatment of cancer.
MethodsTherapy preference scale TPS has a total of 30 questions (Future Oncol 17 (01), 37-44). Nineteen questions measure patients’ rating of importance of safety and quality of life, effectiveness of cancer treatment, and other treatment characteristics on an ordinal scale of 1-10. Eight questions query the importance of treatment aspects with a 4-item Likert scale with choices of 'strongly disagree,’ 'disagree,’ 'agree,’ and 'strongly agree.’ Three questions identify patients’ preferences on intent of therapy (cure, extension of life, or relief of symptoms), acceptable maximum out-of-pocket expenses ($1000, $5000, $10,000, $15,000, or $20,000), and minimum life expectancy gain to accept cancer treatment (3, 6, 9, 12, or 15 months).
Study design and participants We recruited 300 adults >18 years with any cancer type from the University of Nebraska Medical Center.
Data collection and analysis We collected patient-, cancer- and treatment-related data. Patients were categorized into 4 age-by-sex groups (younger men <60 years, older men ≥60 years, younger women <60 years, older women ≥60 years) for analysis. We compared responses to questions on a 10-point Likert scale across the 4 groups with the Kruskal-Wallis non-parametric ANOVA method. Due to the ordinal nature of data, responses to questions on a 4-point Likert scale were recorded as 1 through 4, with strongly disagree=1, disagree=2, agree=3, and strongly agree=4. We compared responses across the groups with the Kruskal-Wallis non-parametric ANOVA method. p-value < 0.05 was considered statistically significant.
ResultsPatient characteristics Patients with hematological malignancies comprised 38% of the total cohort- out of which 63% had myeloid malignancies. Among total patients, 59 were (19.7%) young men, 96 (32%) were older men, 58 were (19.3%) young women, and 87(29%) were older women.
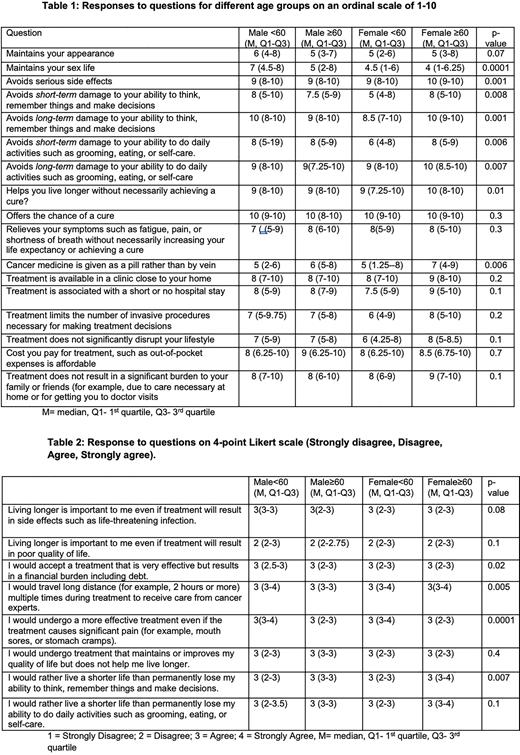
Safety and Quality of life All study participants prioritized cure over prolonging life or symptomatic treatment only (Table 1). However, relief of symptoms such as fatigue, pain, and shortness of breath without necessarily increasing life expectancy or achieving cure was valued. All participants preferred affordable out-of-pocket expenses and less burden on family or friends. There were significant differences in some responses among the 4 age-by-sex groups. Maintaining sex life was more important for younger men than for other groups. Compared to other groups, avoiding long-term impact on daily activities was more important for older women and less important for older men. Older men and older women preferred oral pills to intravenous therapy. Avoiding serious side effects was more important to older women than others. Avoiding short term impact on daily activity and avoiding long-term ability to think were less important to younger women.
Treatment characteristics All participants would avoid life-threatening infection and poor quality of life than living longer. Among the 4 groups, financial debt was less important for younger men, and shorter travel distance was more important for older men (Table 2). Younger men and younger women valued more effective treatment than significant pain (e.g., mouth sores or stomach cramps). Living a shorter life than permanently losing ability to think, remember things, and make decisions was more important for older women than for other groups.
Discussion Our study results indicate that all patients prioritized cure, but relief of symptoms such as fatigue, pain, and shortness of breath was important. Preferences differed in other aspects, such as short-term and long-term effects on daily activities and ability to think, remember and make decisions, oral therapy, financial burden, and sex life. Our findings highlight that understanding patients’ preferences depends on assessing how they view different aspects of treatment with the possible outcomes and goals of care. A multidimensional scale like TPS can help provide valuable insights regarding patients’ preferences to guide appropriate treatment plans.
Disclosures
Vose:Kite, a Gilead Company: Research Funding; Lilly: Honoraria; Janssen: Honoraria; AstraZeneca: Honoraria; Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy; Johnson & Johnson: Consultancy; AbbVie: Honoraria; MorphoSys: Consultancy. Bhatt:Protagonist: Other: Member, Safety Monitoring Committee ; Genentech: Honoraria; Incyte: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Servier Pharmaceuticals: Honoraria; Pfizer: Research Funding; National Marrow Donor Program: Research Funding; Jazz: Research Funding; Chimerix: Other: Drug support for a trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal